Background: Hepatotoxicity is a well-recognised side effect of tyrosine kinase inhibitors (TKI) and in some patients it can be a challenge to find a well-tolerated and effective drug. Isolated hyperbilirubinemia is common but, particularly in the case of nilotinib, is generally attributed to concomitant Gilbert's syndrome, characterized by the reduced expression of uridine diphosphate glucuronosyltransferase 1A1 (UGT1A1). We have previously reported hyperbilirubinemia in association with the homozygous Gilbert genotype in patients treated with imatinib, dasatinib and nilotinib and have now extended our studies to more recent TKI. In addition we have analysed the incidence of TKI-associated transaminitis and attempted to identify safer alternative TKI.
Methods: From our database of >900 patients presenting with CML CP1 from 2000-2022 and treated with a TKI, we identified 467 individuals who had received only TKI (prior interferon, combination experimental therapy, auto- and allografted patients all excluded), had normal liver function tests (LFT) at TKI start and for whom serial consecutive LFT were available. Variations in dinucleotide repeats in the UGT1A1 promoter region were analyzed by PCR with genotypes: 6/6 (homozygous for (TA) 6 allele; wild-type), 7/7 (homozygous for (TA) 7 allele) and 6/7 (heterozygous). Because individual patients could receive more than one TKI, we defined the period on treatment with each TKI as an ‘episode’ so as to attribute abnormal LFT to a specific drug. Abnormal LFT were graded according to the CTCAE version 4.0 and any occurrence of abnormalities was cross-checked to exclude a confounding reason (e.g. antibiotics, cholecystitis). Patients were censored at last LFT.
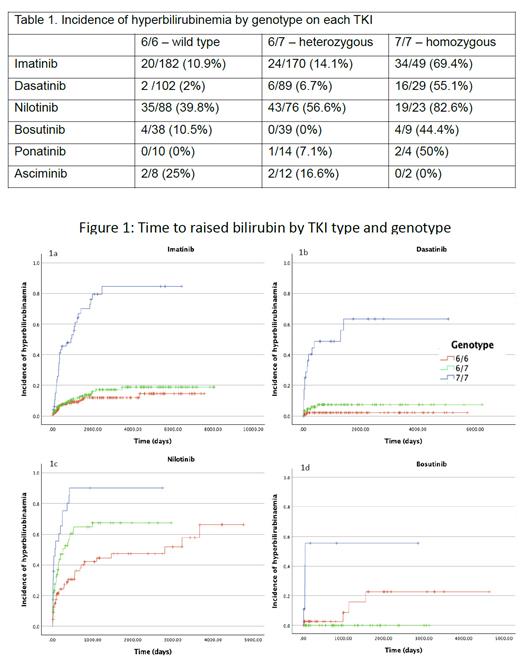
Results: 229 females and 238 males (median age at diagnosis 47 yrs (range 11-90) had 955 separate TKI episodes: imatinib 406, dasatinib 222, nilotinib 189, bosutinib 87, ponatinib 28 and asciminib 23. The median number of LFT per patient was 47 (range 3-264). 7 UGT1A1 genotypes were identified, of which 45%, 41% and 13% were wild type 6/6, heterozygous 6/7 and homozygous 7/7 respectively (6 patients with other genotypes excluded). With the exception of asciminib, hyperbilirubinemia was more frequent in homozygous patients for all TKI. It was also common in all patients treated with nilotinib but in wild type and heterozygous patients, was frequently associated with concomitant transaminitis. A close temporal relationship between start of TKI and raised bilirubin suggests causality (Fig 1a-d).
The incidences of transaminitis of any grade were 44%, 47%, 65%, 66%, 43% and 39%, and of >grade 1 were 7%, 8%, 17%, 32%, 18% and 13% for imatinib, dasatinib, nilotinib, bosutinib, ponatinib and asciminib respectively. Of 202 patients with a transaminitis on first line TKI, 94 were subsequently switched to a 2 nd line TKI, and 27 of those to a 3 rd line TKI. Irrespective of the TKI used, recurrent transaminitis of any grade was common occurring in 60/94 (64%) on 2 nd line and 19/27 (71%) on 3 rd line, although abnormalities >grade 2 were unusual (<1% on 2 nd line, <5% on 3 rd line).
179 patients developed transaminitis on imatinib, 46 and 29 switched (rarely for transaminitis) to dasatinib and nilotinib respectively. 28 (61%) on dasatinib and 23 (79%) on nilotinib had a second transaminitis (dasatinib - 14% >grade 1, nilotinib - 22% >grade 1)). Of 227 patients without transaminitis on imatinib, 63 and 67 switched to dasatinib or nilotinib. 28 (44%) on dasatinib and 38 (57%) on nilotinib developed further transaminitis (dasatinib - 0% >grade 1, nilotinib 19% >grade1).
With respect to identifying a ‘safer’ drug we studied in detail 15 patients with grades 3-4 transaminitis on 1 st line therapy; imatininb-10, dasatinib-1, nilotinib-3, bosutinib -1. Four settled on dose reduction, and of the remaining 11, 7 returned to normal or maximum grade 1 on dasatinib.
Conclusion: With the exception of asciminib the previous association of TKI, homozygous Gilbert and hyperbilirubinemia was confirmed. Grade 1 transaminitis is common on all TKIs, with higher grades of severity most frequent on bosutinib. Patients experiencing abnormal transaminases on 1 st line TKI are likely to have similar problems on 2 nd and 3 rd line agents, with a trend to higher incidence/severity on nilotinib compared to dasatinib.
Disclosures
Milojkovic:Incyte: Honoraria; Novartis: Honoraria; Pfizer: Honoraria. Apperley:Incyte: Honoraria, Research Funding, Speakers Bureau; Pfizer: Research Funding; Novartis: Honoraria, Speakers Bureau; Bristol Myers Squibb: Honoraria, Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal